Comprendre les pesticides et leur cycle dans l’environnement
Les pesticides ont subi leur juste part des abus au cours des années. Certains d’entre eux justifiaient, certains d’entre eux se faisaient passer. En ces jours de médias sociaux et de «nouvelles fausses», quiconque peut faire le plus grand éclaboussement pour Search Engine Optimization (SEO) et des hits uniques sur une page Web ou une vidéo, gagne. D’accord? … uhm … pas tellement.
Le résultat final avec la protection des plantes à l’aide de pesticides réside dans le risque: bénéfice. Le risque étant l’économie, l’efficacité, la persistance du produit chimique dans l’environnement et le danger pour la personne qui l’applique. La récompense étant le rendement économique maximal (en réduisant vos coûts alimentaires), des aliments de meilleure qualité (bactéries et champignons réduits, et une valeur nutritionnelle plus élevée, car la plante est plus saine et son rendement en nourriture) et la fraîcheur des produits.
Les agriculteurs ont largement contribué à intégrer la lutte antiparasitaire (IPM). Connaissez vos récoltes (toutes), explorez vos cultures (et vos champs), répondez à la pression des ravageurs lorsqu’elle atteint le seuil économique en utilisant toutes les méthodes à votre disposition. Il s’agit notamment de pratiques culturelles telles que la rotation et le travail du sol, les cultures de couverture, la culture, le temps de récolte, la connaissance de l’organisme nuisible et son cycle de vie, et plus … avant d’atteindre la «bouteille».
Quel est exactement un pesticide?
Les pesticides comprennent les insecticides, les herbicides, les fongicides et les rodenticides. Chacun a ses propres régimes pour le chronométrage en fonction des ravageurs qu’ils contrôlent.
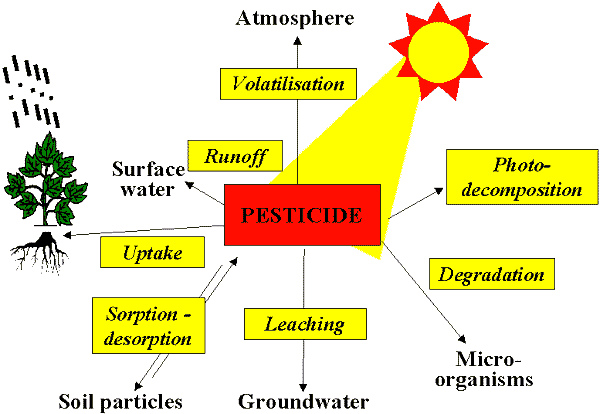
Quel est le destin de l’environnement d’un pesticide après son application?
Sur demande, un ou plusieurs des nombreux destins possibles se produisent à des pesticides. Ceux-ci concernent son mode d’action, ainsi que son impact sur l’environnement.
Absorption is the process of certain pesticides being taken up by the roots, or absorbed in the leaves, and moved through the plant tissue. In soil active herbicides, the plant or germinating seed removes the pesticide from the soil solution, it is translocated through the target, and either kills it, or doesn’t.
Adsorption occurs when the pesticide molecule is bound to the soil particle, organic matter, or other substance, and ‘sticks’ to it through chemical or physical bonding. For example, paraquat, a contact herbicide once commonly used to burn down existing biomass prior to no-till or other reason, binds so tightly to the clay molecules in the soil, it would never enter the soil solution. Hence its only mode of action is contact with live, above ground tissue. Other factors that relate to how well a chemical is bound to the soil relate to pH, texture, and organic matter. When bound to the soil, chances are the pesticide is not likely going to enter the environment. Clay and organic matter tend to tie up materials in the soil because of their negative charge. The only way pesticide molecules will move is if the soil they are bound to moves (erosion, runoff). In time, microbes in the soil will degrade the pesticide through digestion and respiration, further keeping it out of the environment.
Solubility of a pesticide is an important consideration, not only from the environmental implications, but from a mode of action standpoint. Highly soluble materials are good when you have the target actively taking up the material. However, highly soluble materials are more subject to leaching when heavy rains occur, taking them out of the germination zone and rendering them ineffective while also moving them into the subsurface water and eventually, the aquifer. On the other side of the coin, materials that are relatively insoluble may persist for a longer time in the soil, even for seasons and years at a time. For example, Atrazine in corn, when used at high rates, will adversely affect or even kill a crop of alfalfa planted the following year.
Degradation of pesticides begins almost immediately after application. This occurs through respiration, digestion, uptake and conversion, or other means by both the plants and the microbes in the soil. The rate of degradation is important when considering the fate of pesticides in the soil environment. It is from this measure, plus solubility, plus adsorption, plus absorption, that the persistence of a chemical is important. A material that is short lived in the soil is also short lived in its window of efficacy on the target, but renders itself to less toxic forms and compounds at the same time. This persistence is generally categorized as residual activity. If a material has a long residual, it is also more subject to moving offsite by erosion; effecting crops to be planted in the future that are sensitive to it. A highly soluble material with a long residual has the greatest potential for leaching to ground water.
As new Plant Protection Materials enter the market, they are designed to combine a short persistence in the environment but a high degree of efficacy on the target they are being applied to. Much of this technology moves from relying heavily on soil active, pre-emergent materials to contact or leaf absorbed materials applied after the weed emerges, or post-emergent materials. An example is glyphosate (Roundup and others). Glyphosate is only absorbed by the leaves. It is highly soluble, but also very positive in charge, so when applied, and it hits the soil, it is tied up so tightly, plants do not take it up through the roots; nor does it move through the soil water to the aquifer.

Although herbicides are usually applied with boom sprayers, consider banding herbicides. Banding saves time and reduces the amount of herbicide used. Source: Weed Control: More than Chemicals and Cultivators. M. Betts,
British Columbia Ministry of Agriculture, Food and Fisheries.
The Future of Chemical Plant Protection
The primary focus points of future technologies in Plant Protection are focused on these primary goals:
- Short lived in the environment
- Efficacy against the target
- Resistance management
- Minimal residual and carryover
The development of genetically modified organisms (GMO’s) plays a role in reducing the environmental impact of plant protection materials, as well as reducing their total use in many cases. The Agricultural Industry worldwide is focused on reducing the impact of its activities on the environment as much or more than any other industry.
As one farmer put it to a group at a meeting I attended once on groundwater in Agriculture, “Hey, don’t forget my wife pulls that water from our well every day!”.
Integrating pest management using all the tools and knowledge available across cultural, economic, AND chemical options will continue to drive the efficient food systems being developed across the world while sustaining our planet for generations to come.
Read more about it:
Sources used in this article, and some others that you might find interesting include:
Understanding the Fate of Pesticides after Application – Pesticide Environmental Stewardship and Cornell University
Pesticides in the Nation’s Streams and Ground Water – United States Geological Survey (USGS) – Science for a Changing World
What Happens to Pesticides Released in the Environment? – National Pesticide Information Center
By: John Deibel, Freelance Writer
Do you liked this post..? Help us by sharing it on Facebook or Google+